Aluminium Kit
how to test |
|
|
| Water Test Kit (2-test pack) |
| Adrenal Function Urine Test |
| Sulkowitch Urine (Calcium) Test |
|
|
|
ALUMINUM or ALUMINIUM
Number 13 on the "periodic table" of elements
Pure aluminum is a silvery-white metal with many desirable characteristics. It is light, nontoxic (as the metal), nonmagnetic and
nonsparking. It is somewhat decorative and easily formed, machined, and cast. Pure aluminum is soft and lacks strength, but alloys with
small amounts of copper, magnesium, silicon, manganese, and other elements have very useful properties. Aluminum is an abundant element in
the earth's crust, but it is not found free in nature.
Aluminum is used in:
- cans and foils
- kitchen utensils
- outside building decoration
- industrial applications where a strong, light, easily constructed material is needed
although its electrical conductivity is only about 60% that of copper per area of cross section, it is used in electrical transmission lines
because of its lightness and price
- alloys are of vital importance in the construction of modern aircraft and rockets
aluminum, evaporated in a vacuum, forms a highly reflective coating for both visible light and radiant heat. These coatings soon form a thin
layer of the protective oxide and do not deteriorate as do silver coatings. These coatings are used for telescope mirrors, decorative paper,
packages, toys, and in many other uses
- the oxide, alumina, occurs naturally as ruby, sapphire, corundum, and emery, and is used in glass making and refractories. Synthetic ruby
and sapphire are used in the construction of lasers
The amount of aluminum in the human body ranges from 50 to 150 mg., with an average of about 65 mg. Most of this metal is
found in the lungs, brain, kidneys, liver and thyroid. Our daily intake ranges from 10 to 110 mg but the body will eliminate
most of this in our feces, urine and some in sweat.
Toxicity
Aluminum is probably the least toxic of the metals, although the concern is that it has become so pervasive and is now found in higher levels
in human tissues. It is not clear how aluminum interferes with activities in the human body. It may reduce vitamin levels or bind to DNA, and
it has been correlated with weakened tissue of the gastrointestinal tract. In Alzheimer's disease, there are increased aluminum levels in the
brain tissue and an increase in what are called "neurofibrillary tangles," which tend to reduce nerve synapses and conduction.
Oral aluminum, as obtained from antacids, can bind pepsin and weaken protein digestion. It also has astringent qualities, and therefore can
dry the tissues and mucous linings and contribute to constipation. Regular use of aluminum-containing deodorants may contribute to the
clogging of underarm lymphatics and then to breast problems such as cystic disease.
Effects of Aluminum
Acute aluminum poisoning has been associated with constipation, colicky pain, anorexia, nausea and gastrointestinal irritation, skin problems,
and lack of energy. Slower and longer-term increases in body aluminum may create muscle twitching, numbness, paralysis, and fatty
degeneration of the liver and kidney.
It is worse with reduced renal function. Aluminum may reduce the absorption of selenium and phosphorus from the gastrointestinal tract. The
loss of bone matrix from aluminum toxicity can lead to osteomalacia, a softening of the bone. Skin rashes have occurred with local irritation
from aluminum antiperspirants.
Aluminum toxicity has been implicated in the brain aging disorders. Alzheimer's disease and parkinsonism have both become more prevalent as
the incidence of aluminum toxicity has increased. Areas with high amounts of aluminum in the drinking water are showing an increase in the
incidence of Alzheimer's disease (alum and aluminum sulfate are used to treat water in many cities). Although increased aluminum has been
measured in the brain and other body tissues in Alzheimer's diseases, other factors may be contributing as well. There seems to be a
weakening of the blood-brain barrier in Alzheimer's disease, and this may allow a variety of brain toxins to reach the central nervous system.
What is causing this breakdown of the barrier between the brain and the rest of the body is not yet clear. It is also important to examine
aluminum toxicity in children with hyperactivity and learning disorders, as it has been implicated in these problems.
Toxicity Limits
Organic - A reading under 15-20 ppm is considered normal, but 10-15 ppm, is probably ideal.
Inorganic - Should be 0 ppm
Check out the aluminum in your body with our easy to use, home-based, HMT Aluminum Test Kit
 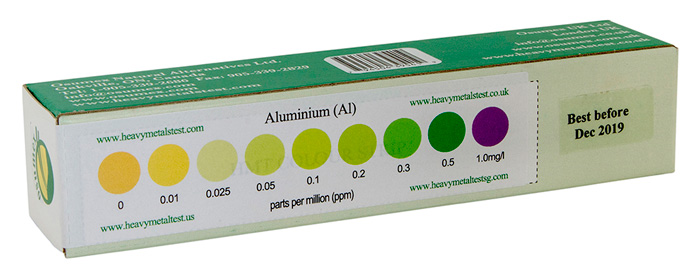
Sample of a HMT Aluminum Test kit with color strip for results analysis
Osumex HM-Chelat is most effective in eliminating heavy metals contamination in the body

The above information is provided for general
educational purposes only. It is not intended to replace competent
health care advice received from a knowledgeable healthcare professional.
You are urged to seek healthcare advice for the treatment of any
illness or disease.
Health Canada and the FDA (USA) have not evaluated these
statements. This product is not intended to diagnose, treat, cure, or prevent
any disease.
|


